
Location: Istituto di BioRobotica – Scuola Superiore Sant’Anna
Lab head: Prof. Matteo Cianchetti
Contact: matteo.cianchetti@santannapisa.it
Lab Team
Lab Mission
The laboratory is a complete facility for the study of new concept, devices and prototypes that address diseases related to the human cardio-circulatory system. The facility has a complete set of assessment modules that allow the evaluation of research prototypes and systems under physio-pathological conditions. A non-exhausting list of tested devices: cardiac devices (total artificial heart or single ventricles), artificial heart valves, vascular grafts, interventional instruments for MIS, (externally guided) endoluminal navigation, drug release/delivery.
Devices
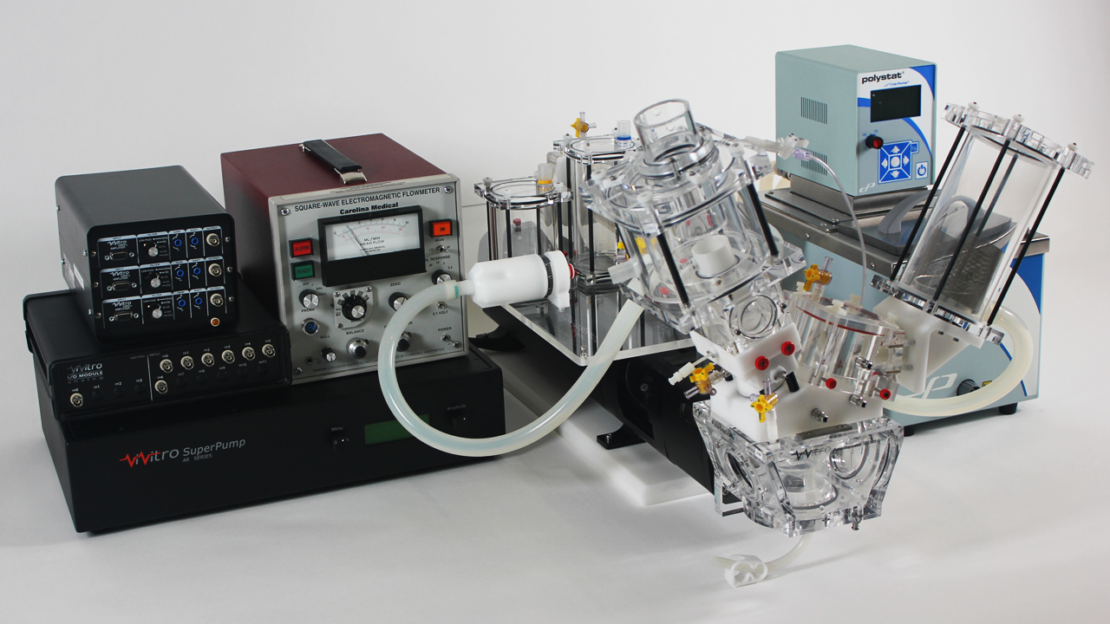
Pulse Duplicator
The Pulse Duplicator System assesses the performance of prosthetic heart valves under simulated cardiac conditions. The Pulse Duplicator simulates physiological or other complex flow variations while allowing the user to vary the peripheral resistance and compliance of the system. Pressure and flow can be measured from aortic or mitral sites. It complies with ISO 5840:2021 requirements.
Features:
- Controls pulsatile fluid flows to simulate various cardiac flow conditions
- Ventricle membrane to replicate the natural chamber flow
- Simulates cardiovascular peripheral resistance and compliance
- Certificated with St. Jude Master’s Valve test method results
- Flow readings can be collected in real-time, meeting ISO 5840 requirements
- Flow can be measured directly at the aortic or mitral valve positions
- Flow can be measured directly at the pulmonary and tricuspid valve positions
- Pressure readings can be collected in real-time, meeting ISO 5840 requirements
- Measurement of wall pressures in the atrium, ventricle, and aortic outflow tract
- Acrylic windows permit echo imaging, Doppler flow measurements, and PIV
Applications:
- Mechanical, polymeric, and biological surgical valve prosthesis
- Polymeric and biological valve prosthesis for Transcatheter Aortic Valve Replacement (TAVR)
- Polymeric and biological valve prosthesis for Transcatheter Aortic Valve Implantation (TAVI)
- Polymeric and biological valve prosthesis for Transcatheter Tricuspid Valve Replacement (TTVR)
- Polymeric and biological valve prosthesis for Transcatheter Mitral Valve Replacement (TMVR)
- Stentless valves
- Structural heart and peripheral vascular devices
- Occluder and Closure Devices (with customization)
- Evaluate LVAD or other assist devices (with customization)
- Advanced cardiac flow loop systems for pressure and flow monitoring devices
Data sheet: download
Note: Usable only with technical supervision
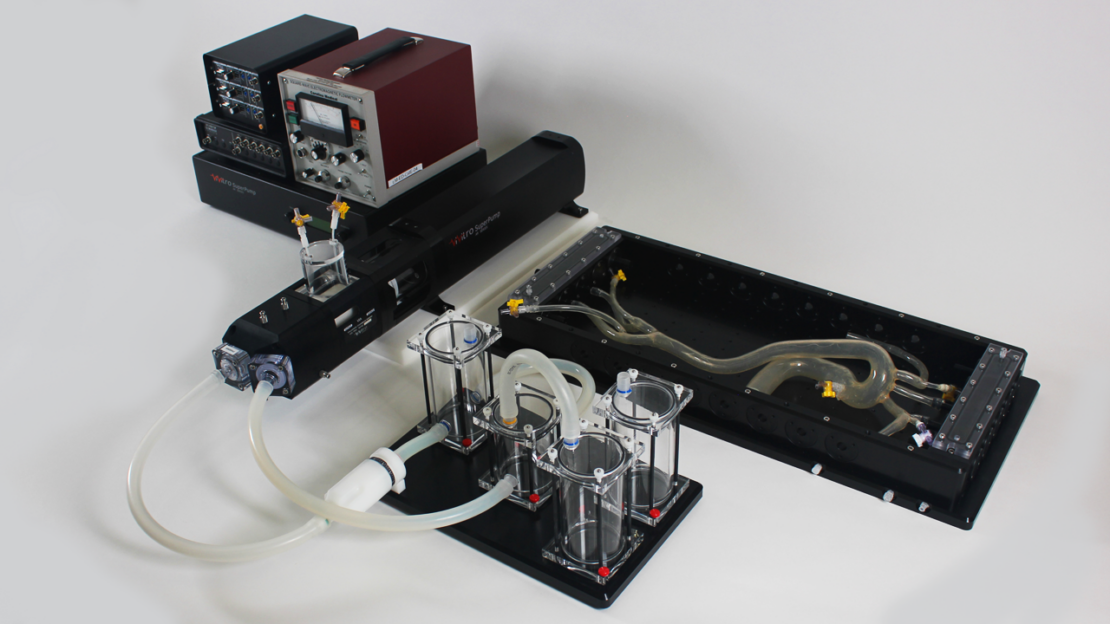
Endovascular Simulator
The Endovascular Simulator is a platform with characteristics of modularity and flexibility of use. Indeed, several cardiovascular devices and prostheses can be tested, replicating a realistic scenario of anatomy and fluid flow conditions. The Endovascular Simulator addresses requirements of ISO 5840, ISO 25539, and ISO 7198 standards as they include simulated use guidelines.
Features:
- Controls pulsatile fluid flows to simulate various cardiac flow conditions
- Simulates cardiovascular peripheral resistance and compliance
- Flow readings can be collected in real-time, meeting ISO 5840 requirements
- Flow can be measured directly in the selected vascular site
- Pressure readings can be collected in real-time, meeting ISO 5840 requirements
- Ventricle membrane to replicate the natural chamber flow
Applications:
- Heart valve function testing
- Peripheral devices
- Vascular devices
- Aortic Valve By-Pass
- LVADs simulator
- Flow transducer evaluation/calibration
- Isolated heart driver
- Echo Doppler flow phantom
- Neurovascular studies
- Blood vessel flow studies
- MRI studies
- Bioreactors & Incubators
- Applications requiring physiological flows and pressure
- Other cardiovascular devices
Data sheet: download
Note: Usable only with technical supervision





